Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
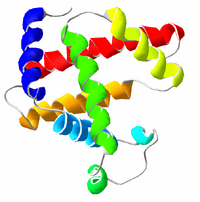
A representation of the 3D structure of myoglobin, showing coloured alpha helices. This protein was the first to have its structure solved by X-ray crystallography by Max Perutz and Sir John Cowdery Kendrew in 1958, which led to their receiving a Nobel Prize in Chemistry.
A protein (from the Greek protas meaning "of primary importance") is a complex, high-molecular-weight organic compound that consists of amino acids joined by peptide bonds. Proteins are essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes, catalyzing chemical reactions. Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton, serving as biological scaffolds for the mechanical integrity and tissue signalling functions. Still more functions filled by proteins include immune response and the storage and transport of various ligands. In nutrition, proteins are broken down through digestion, which begins in the stomach. There proteins are broken down into proteoses and polypeptides to provide amino acids for the organism, including those the organism may not be able to synthesise itself. Pepsinogen is converted into the enzyme pepsin when it comes into contact with hydrochloric acid. Pepsin is the only proteolytic enzyme that digests collagen, the major protein of connective tissue. Most protein digestion takes place in the duodenum with the overall contribution from the stomach being small. Almost all protein is absorbed when it reaches the jejunum with only 1% of ingested protein left in the feces. Some amino acids remain in the epithelial cells and are used for synthesis of new proteins, including some intestinal proteins, constantly being digested, recycled and absorbed from the small intestine.
Proteins are one of the classes of bio-macromolecules, alongside polysaccharides, lipids, and nucleic acids, that make up the primary constituents of living things. They are among the most actively-studied molecules in biochemistry, and were discovered by Jöns Jakob Berzelius in 1838.
The coding sequences of genes determine the amino-acid sequences of almost all naturally occurring proteins, via the processes of transcription and translation. In many cases, the resulting protein is then chemically altered (post-translational modification), before becoming functional. It is very common for proteins to work together to achieve a particular function, and often physically associate with one another to form a complex.
Properties of protein
Components and synthesis
Main articles: Amino acid and Protein biosynthesis
Proteins are biopolymers built from 20 different L-alpha-amino acids. Proteins are assembled from amino acids using information present in genes. Genes are transcribed into RNA, RNA is then subject to post-transcriptional modification and control, resulting in a mature mRNA that undergoes translation into a protein. mRNA is translated by ribosomes that match the three-base codons of the mRNA to the three-base anti-codons of the appropriate tRNA. The enzyme aminoacyl tRNA synthetase catalyzes the formation of covalent peptide bonds between amino acids forming the protein.
The two ends of the amino acid chain are referred to as the carboxy terminus (C-terminus) and the amino terminus (N-terminus) based on the nature of the free group on each extremity.
Structure
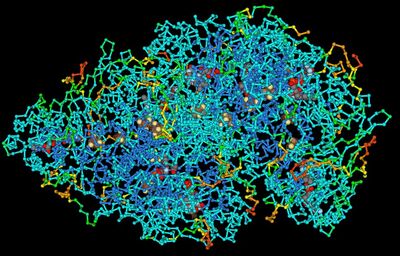
Example of 3-dimensional structure of protein
Proteins fold into unique 3-dimensional structures. The shape into which a protein naturally folds is known as its native state, which is determined by its sequence of amino acids. Thus, proteins are their own polymers, with amino acids being the monomers. Biochemists refer to four distinct aspects of a protein's structure:
- Primary structure: the amino acid sequence
- Secondary structure: highly patterned sub-structures — alpha helix and beta sheet — or segments of chain that assume no stable shape and are formed by hydrogen bonding. Secondary structures are locally defined, meaning that there can be many different secondary motifs present in one single protein molecule.
- Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structural motifs to one another; primarily formed by hydrophobic interactions, but hydrogen bonds, ionic interactions, and disulfide bonds are usually involved too.
- Quaternary structure: the shape or structure that results from the union of more than one protein molecule, usually called protein subunits in this context, which function as part of the larger assembly or protein complex.
In addition to these levels of structure, proteins may shift between several similar structures in performing their biological function. In the context of these functional rearrangements, these tertiary or quaternary structures are usually referred to as " conformations," and transitions between them are called conformational changes.
The process by which the higher structures are formed is called protein folding and is a consequence of the primary structure. The mechanism of protein folding is not entirely understood. Although any unique polypeptide may have more than one stable folded conformation, each conformation has its own biological activity and only one conformation is considered to be the active, or native conformation.
Protein regulation
Various molecules and ions are able to bind to specific sites on proteins. These sites are called binding sites. They exhibit chemical specificity. The particle that binds is called a ligand. The strength of ligand-protein binding is a property of the binding site known as affinity.
Since proteins are involved in practically every function performed by a cell, the mechanisms for controlling these functions therefore depend on controlling protein activity. Regulation can involve a protein's shape or concentration. Some forms of regulation include:
- Allosteric modulation: When the binding of a ligand at one site on a protein affects the binding of ligand at another site.
- Covalent modulation: When the covalent modification of a protein affects the binding of a ligand or some other aspect of the protein's function.
Diversity
Proteins are generally large molecules, having molecular masses of up to 3,000,000 (the muscle protein titin has a single amino acid chain 27,000 subunits long) however protein masses are generally measured in kiloDaltons (kDa). Such long chains of amino acids are almost universally referred to as proteins, but shorter strings of amino acids are referred to as "polypeptides," "peptides" or rarely, "oligopeptides". The dividing line is undefined, though "polypeptide" usually refers to an amino acid chain lacking tertiary structure which may be more likely to act as a hormone (like insulin), rather than as an enzyme (which depends on its defined tertiary structure for functionality).
Proteins are generally classified as soluble, filamentous or membrane-associated (see integral membrane protein). Nearly all the biological catalysts known as enzymes are soluble proteins. Antibodies, the basis of the adaptive immune system, are another example of soluble proteins. Membrane-associated proteins include exchangers and ion channels, which move their substrates from place to place but do not change them; receptors, which do not modify their substrates but may simply shift shape upon binding them. Filamentous proteins make up the cytoskeleton of cells and much of the structure of animals: examples include tubulin, actin, collagen and keratin, all of which are important components of skin, hair, and cartilage. Another special class of proteins consists of motor proteins such as myosin, kinesin, and dynein. These proteins are "molecular motors," generating physical force which can move organelles, cells, and entire muscles.
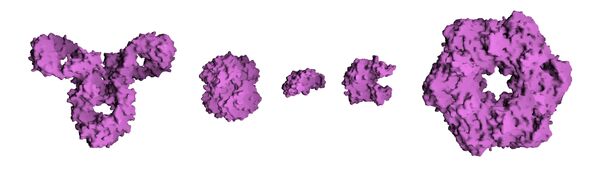
Molecular surface of several proteins showing their comparative sizes. From left to right are: Antibody (IgG), Hemoglobin, Insulin (a hormone), Adenylate Kinase (an enzyme), and Glutamine Synthetase (an enzyme).
Role of protein
Functions
Proteins are involved in practically every function performed by a cell, including regulation of cellular functions such as signal transduction and metabolism. Life, chemically speaking, is nothing but the function of proteins although the information to make a unique protein resides in DNA. The protein involved in functions control almost all the molecular processes of the body. Without such proteins, the activity requires a different set of conditions, such as high temperature and pressure. Functional proteins are those molecules that do everything that happens within us. For example, protein catabolism requires enzymes termed proteases and other enzymes such as glycosidases.
Nutrition
Benefits in the diet
Protein is an important macronutrient to the human diet, supplying the body's needs for nitrogen and amino acids, the building blocks of proteins. Mammals cannot synthesize all 20 amino acids, so protein from the diet is necessary for life and the amino acids that cannot be synthesized by the body are known as essential amino acids. The exact amount of dietary protein needed to satisfy these requirements, known as an RDA may vary widely depending on age, sex, level of physical activity, and medical condition.
Protein deficiency can lead to symptoms such as fatigue, insulin resistance, hair loss, loss of hair pigment, loss of muscle mass, low body temperature, hormonal irregularities, as well as loss of skin elasticity. Severe protein deficiency, encountered only in times of famine, is fatal, due to the lack of material for the body to facilitate as energy.sport_nutrition
Potential risks
Some suspect excessive protein intake is linked to several problems;
- Overreaction within the immune system
- Liver dysfunction due to increased toxic residues
- Loss of bone density, frailty of bones is due calcium and glutamine being leached from bone and muscle tissue to balance increased acid intake from diet (blood pH is maintained at around 7.4). This effect is not present if intake of alkaline minerals (from fruits and vegetables, cereals are acidic as are proteins, fats are neutral) is high. In such cases, protein intake is anabolic to bone. [1]
It is assumed by researchers in the field, that excessive intake of protein forces increased calcium excretion. If there is to be excessive intake of protein, it is thought that a regular intake of calcium would be able to stablilise, or even increase the uptake of calcium by the small intestine, which would be more beneficial in older women protein_impact.
Proteins are often progenitors in allergies and allergic reactions to certain foods. This is because the structure of each form of protein is slightly different; some may trigger a response from the immune system while others remain perfectly safe. Many people are allergic to casein, the protein in milk; gluten, the protein in wheat and other grains; the particular proteins found in peanuts; or those in shellfish or other seafoods. It is extremely unusual for the same person to adversely react to more than two different types of proteins, due to the diversity between protein or amino acid types.[How to reference and link to summary or text]
Studying proteins
Proteins are sensitive to their environment. They may only be active in their native state, over a small pH range, and under solution conditions with a minimum quantity of electrolytes. A protein in its native state is often described as folded. A protein that is not in its native state is said to be denatured. Denatured proteins generally have no well-defined secondary structure. Many proteins denature and will not remain in solution in distilled water.
One of the more striking discoveries of the 20th century was that the native and denatured states in many proteins were interconvertible, that by careful control of solution conditions (by for example, dialyzing away a denaturing chemical), a denatured protein could be converted to native form. The issue of how proteins arrive at their native state is an important area of biochemical study, called the study of protein folding.
Through genetic engineering, researchers can alter the sequence and hence the structure, "targeting", susceptibility to regulation and other properties of a protein. The genetic sequences of different proteins may be spliced together to create "chimeric" proteins that possess properties of both. This form of tinkering represents one of the chief tools of cell and molecular biologists to change and to probe the workings of cells. Another area of protein research attempts to engineer proteins with entirely new properties or functions, a field known as protein engineering.
Protein-protein interactions can be screened for using two-hybrid screening.
History
The first mention of the word protein, which means of first rank, were from a letter sent by Jöns Jakob Berzelius to Gerhardus Johannes Mulder on 10. July 1838, where he wrote:
- «Le nom protéine que je vous propose pour l’oxyde organique de la fibrine et de l’albumine, je voulais le dériver de πρωτειοξ, parce qu’il paraît être la substance primitive ou principale de la nutrition animale.»
Translated as:
- "The name protein that I propose for the organic oxide of fibrin and albumin, I wanted to derive from [the Greek word] πρωτειοξ, because it appears to be the primitive or principal substance of animal nutrition."
Investigation of proteins and their properties had been going on since about 1800 when scientists were finding the first signs of this, at the time, unknown class of organic compounds.
See also
- Biochemistry
- Crystallography
- Denatured protein
- Intein
- List of recombinant proteins
- List of proteins
- Peptide
- Prion
- Proteinoid
- Protein structure prediction
- Protein targeting
- Proteome
- Ribosome
- Standard curve
- Structural genomics
References
- ^ Kerstetter, J. E., O'Brien, K. O., Insogna, K. L. (2003) "Dietary protein, calcium metabolism, and skeletal homeostasis revisited". J Clin Endocrinol Metab Vol 78, p584S-592 S.
- ^ Kerstetter, J. E., O'Brien, K. O., Caseria, D.M, Wall, D. E. & Insogna, K. L (2005) "The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women". J Clin Endocrinol Metab (2005) Vol 90, p26-31, Entrez PubMed 15546911 15546911.
- ^ Devine, A., Dick, I. M,, Islam I. M., Dhaliwal, S. S. & Prince, R. L. (2005) "Protein consumption is an important predictor of lower limb bone mass in elderly women". Am J Clin Nutr (2005) volume 81 pages 423-428, Entrez PubMed 15941897 15941897.
- ^ Jeukendrup, A. & Gleeson, M. (2004) Sport Nutrition - An Introduction to Energy Production and Performance USA: Human Kinetics
- ^ Bean, A. (2004) Sport Nutrition for Serious Athletes London: Routledge
External links
- The Protein Databank
- UniProt the Universal Protein Resource
- Human Protein Atlas
- iHOP - Information Hyperlinked over Proteins
- Proteins: Biogenesis to Degradation - The Virtual Library of Biochemistry and Cell Biology
- MIT's Laboratory for Protein Molecular Self-Assembly
- Numerous publications on synthetic biomimetic protein-based biomaterials
- Protein Research: Western Blot Protocols, Troubleshooting and Theory
- Amino acid metabolism
- Protein Images
Proteins |
|---|
|
Protein biosynthesis - Posttranslational modification - Protein folding - Protein structure - Protein structural domains - Protein targeting - Proteome - Protein methods - Proteasome List of types of proteins - List of proteins - Membrane protein - Globular protein - Fibrous protein |
ar:بروتين zh-min-nan:Nn̄g-pe̍h-chit ca:Proteïna cs:Bílkovina da:Protein de:Protein et:Valk es:Proteína eo:Proteino fr:Protéine gl:Proteína ko:단백질 io:Proteino id:Protein he:חלבון lv:Olbaltumviela lt:Baltymas lb:Protein hu:Fehérje mk:Протеин nl:Proteïne pt:Proteína ru:Белки simple:Protein sk:Bielkovina sl:Beljakovina sr:Протеин su:Protéin fi:Proteiini sv:Protein ta:புரதம் th:โปรตีน vi:Protein zh:蛋白质 pam:Protina
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |